is hydrogen sulfide polar or nonpolar Polar h2s nonpolar sulfide hydrogen non
H2S (hydrogen sulfide) is a fascinating chemical compound that has garnered a lot of attention in recent times. In this post, we will explore whether H2S is polar or nonpolar and also delve into its Lewis structure.
Is H2S Polar or Nonpolar?
Before we answer this question, let’s understand what we mean by “polar” and “nonpolar” molecules. In chemistry, the polarity of a molecule refers to the distribution of its electron density. A molecule is considered polar if there is an uneven distribution of electrons, resulting in the molecule having a positive and a negative end. Conversely, a molecule is nonpolar if the distribution of electrons within the molecule is even, and there is no separation of charges.
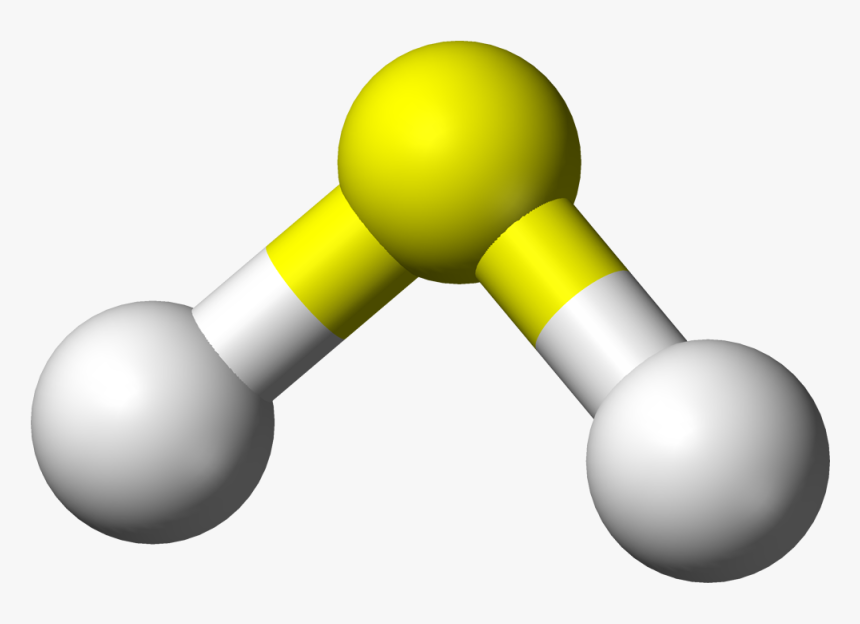
 Now, let’s apply this knowledge to H2S. Hydrogen sulfide (H2S) is a covalent compound that consists of two hydrogen atoms bonded to a sulfur atom. The electronegativity of sulfur is higher than that of hydrogen, which means that sulfur attracts the shared electrons in the covalent bonds towards itself more strongly than hydrogen does.
Now, let’s apply this knowledge to H2S. Hydrogen sulfide (H2S) is a covalent compound that consists of two hydrogen atoms bonded to a sulfur atom. The electronegativity of sulfur is higher than that of hydrogen, which means that sulfur attracts the shared electrons in the covalent bonds towards itself more strongly than hydrogen does.
As a result, the sulfur atom becomes slightly negatively charged, while the hydrogen atoms become slightly positively charged. This uneven distribution of charges in the molecule makes H2S polar. Therefore, we can conclude that H2S is a polar molecule.
H2S Lewis Structure
The Lewis structure of a molecule is a way to represent the valence electrons of its atoms and the bonds between them. It helps us understand the connectivity and arrangement of atoms in a molecule. Let’s take a look at the Lewis structure of H2S, also known as dihydrogen sulfide.
 In the Lewis structure of H2S, the sulfur atom is represented by the letter ‘S,’ and the hydrogen atoms are represented by ‘H.’ The dots surrounding the letters represent the valence electrons of each atom. In H2S, the sulfur atom has six valence electrons, and each hydrogen atom has one valence electron.
In the Lewis structure of H2S, the sulfur atom is represented by the letter ‘S,’ and the hydrogen atoms are represented by ‘H.’ The dots surrounding the letters represent the valence electrons of each atom. In H2S, the sulfur atom has six valence electrons, and each hydrogen atom has one valence electron.
The Lewis structure of H2S reveals that the sulfur atom shares two electrons with each hydrogen atom, resulting in a total of four shared electrons. Additionally, the sulfur atom has two lone pairs of electrons that are not involved in any bond formation.
The Lewis structure of H2S clearly illustrates the polar nature of this molecule. The lone pairs of electrons on the sulfur atom create an uneven distribution of charge, making H2S polar.
In conclusion, H2S (hydrogen sulfide) is a polar molecule due to the uneven distribution of charges caused by the electronegativity difference between sulfur and hydrogen. Its Lewis structure further confirms its polar nature. These characteristics play a significant role in the chemical properties and interactions of H2S in various contexts.
If you are looking for Is H2S ( hydrogen sulfide ) polar or nonpolar you’ve visit to the right page. We have 5 Pics about Is H2S ( hydrogen sulfide ) polar or nonpolar like Is Hydrogen Sulfide a Polar or Nonpolar Molecule, Hydrogen Sulfide - Hydrogen Sulfide Polar, HD Png Download - kindpng and also Hydrogen Sulfide - Hydrogen Sulfide Polar, HD Png Download - kindpng. Read more:
Is H2S ( Hydrogen Sulfide ) Polar Or Nonpolar
 www.bengislife.comh2s polar nonpolar sulfide hydrogen
www.bengislife.comh2s polar nonpolar sulfide hydrogen
Is Hydrogen Sulfide A Polar Or Nonpolar Molecule
 bridgerdesnhbuckley.blogspot.comHydrogen Sulfide - Hydrogen Sulfide Polar, HD Png Download - Kindpng
bridgerdesnhbuckley.blogspot.comHydrogen Sulfide - Hydrogen Sulfide Polar, HD Png Download - Kindpng
 www.kindpng.comsulfide hydrogen kindpng
www.kindpng.comsulfide hydrogen kindpng
H2S Lewis Structure (Dihydrogen Sulfide) In 2021 | Lewis, Chemical
 in.pinterest.comh2s sulfide dihydrogen formula hydrogen sulfur youtu
in.pinterest.comh2s sulfide dihydrogen formula hydrogen sulfur youtu
Is H2S ( Hydrogen Sulfide ) Polar Or Nonpolar
 www.bengislife.compolar h2s nonpolar sulfide hydrogen non
www.bengislife.compolar h2s nonpolar sulfide hydrogen non
Is h2s ( hydrogen sulfide ) polar or nonpolar. H2s polar nonpolar sulfide hydrogen. Is hydrogen sulfide a polar or nonpolar molecule